
Schematic Representation Of Atoms Model Making School Project Bohr
Drawing Bohr-Rutherford diagrams is super easy using the following steps: Find the number of protons, neutrons and electrons for the atom. The number of protons is the atomic number. The number of neutrons can be found by subtracting the number of protons from the atomic mass rounded to the nearest whole. This is because protons and neutrons.
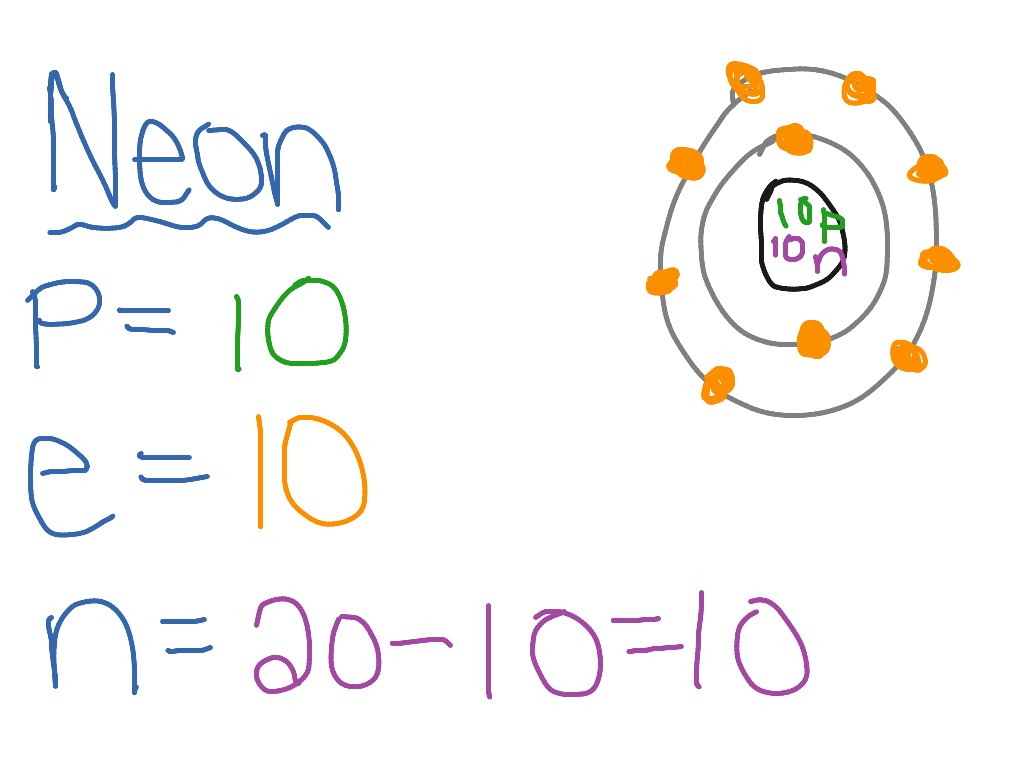
Neon Bohr model Science ShowMe
Atomic Structure (Bohr Model) for the Neon (Ne) Atom Wayne Breslyn 718K subscribers Join Subscribe Subscribed 35 Share 5.7K views 1 year ago In this video we'll look at the atomic structure and.
Bohr Model Neon
Rutherford-Bohr diagram Lewis notation If an element has more than four valence electrons, they are doubled to form pairs of dots around the element. For example, neon is represented by eight valence electrons. Rutherford-Bohr diagram Lewis notation SAMPLE QUESTIONS: 1) Draw a Lewis structure for each of the following elements: Potassium
Bohr Model Neon
The simplest example of the Bohr Model is for the hydrogen atom (Z = 1) or for a hydrogen-like ion (Z > 1), in which a negatively charged electron orbits a small positively charged nucleus. Electromagnetic energy will be absorbed or emitted if an electron moves from one orbit to another. Only certain electron orbits are permitted.

Bohrs atom model Bohrs atom model The neon
The drawbacks of the Rutherford model of the atom, including its apparent instability, would soon be addressed by his student, Dutch physicist Neils Bohr (1885 - 1962). To unlock this lesson you.
Bohr Model Labeled
1). You can effortlessly find every single detail about the elements from this single Interactive Periodic table. 2). You will get the detailed information about the periodic table which will convert a newbie into pro. 3). You will also get the HD images of the Periodic table (for FREE).

Electron Configuration for Neon (Ne) Full Explanation
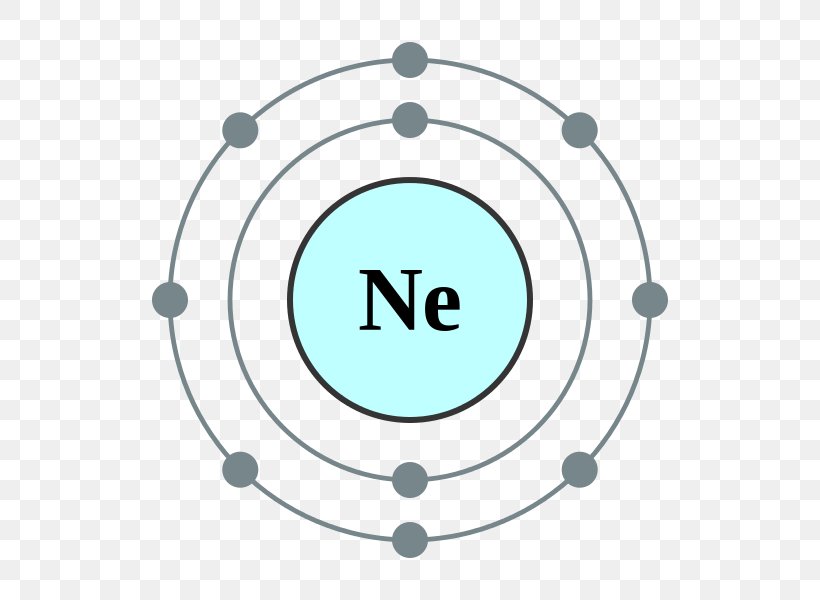
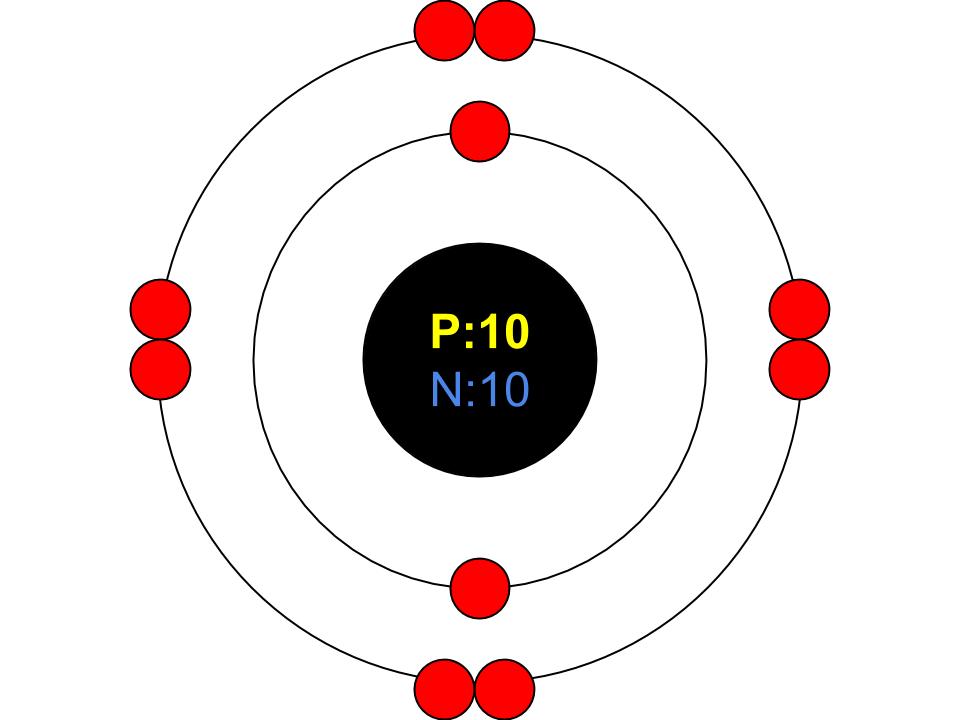
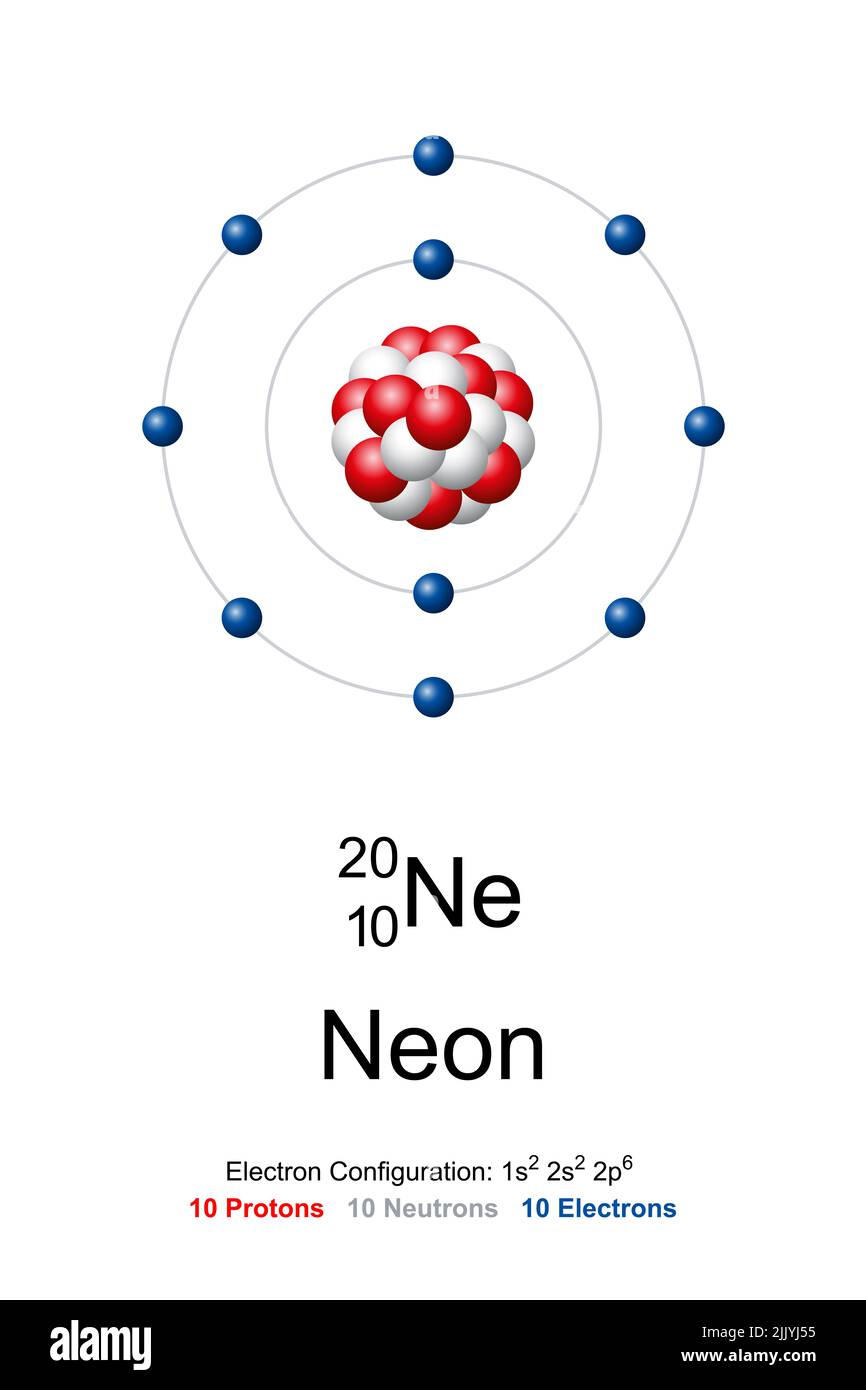
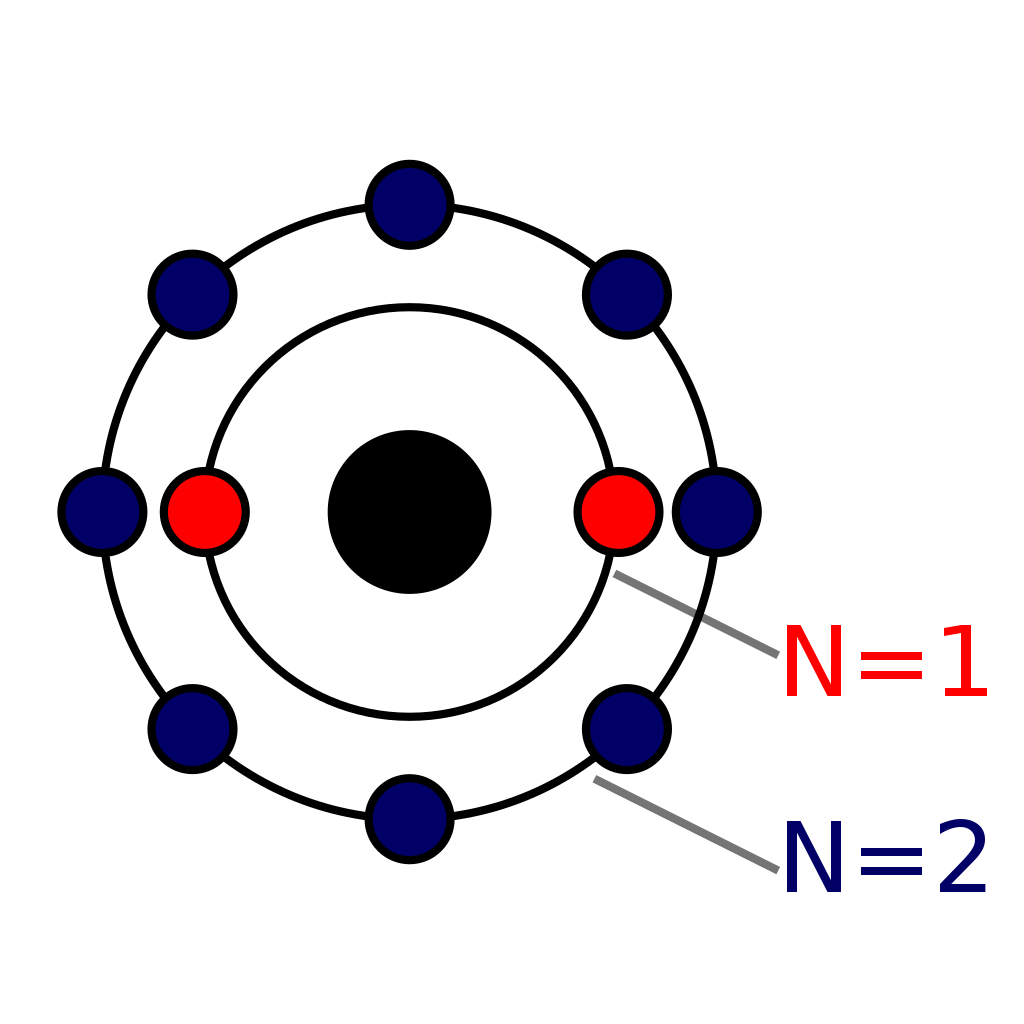
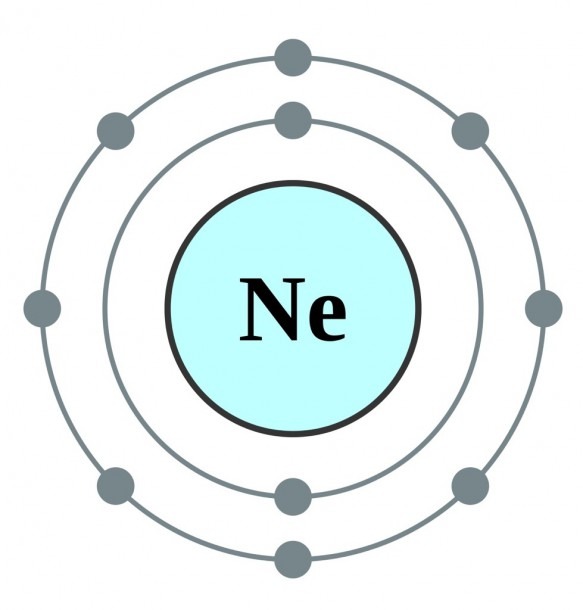
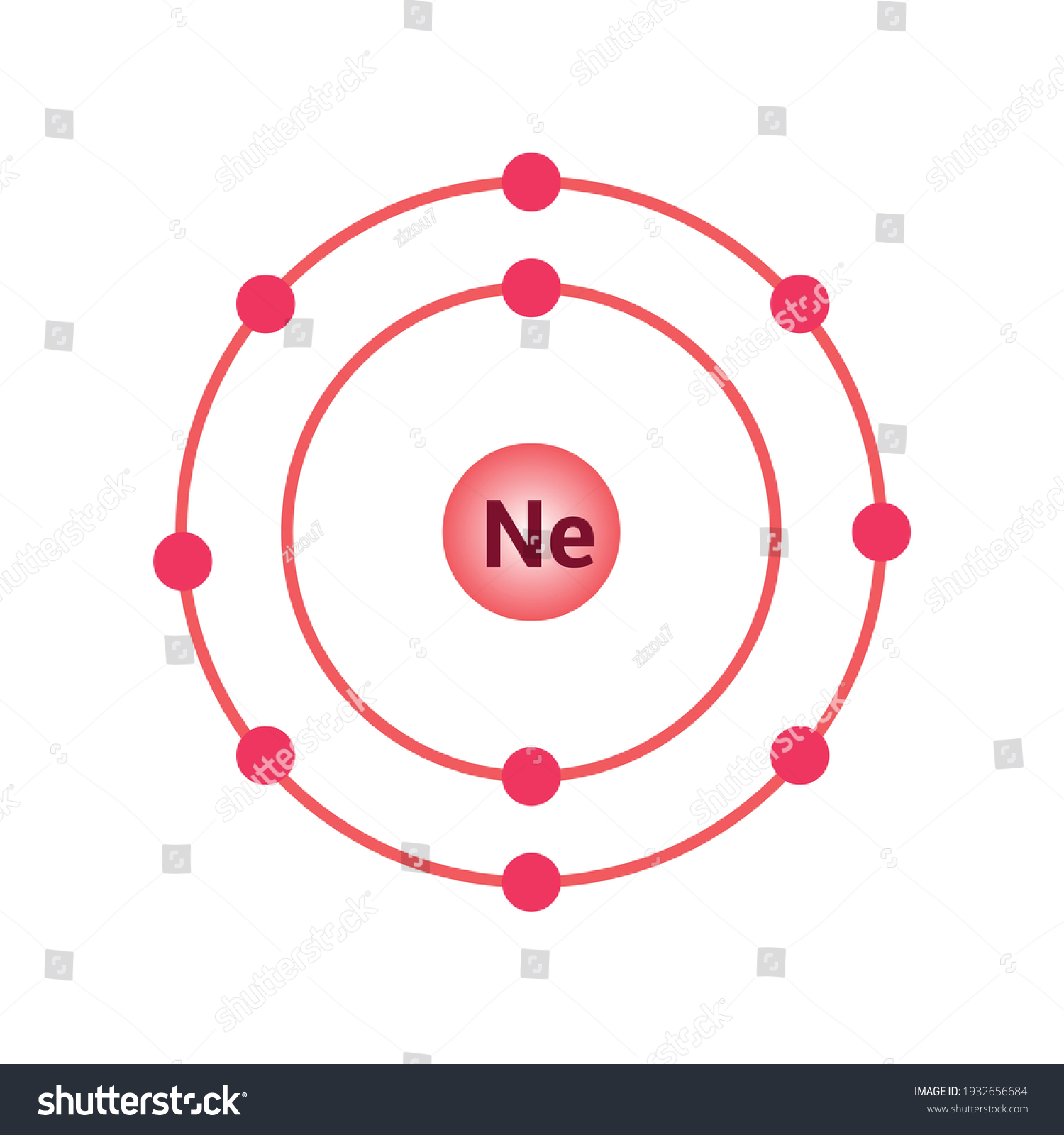
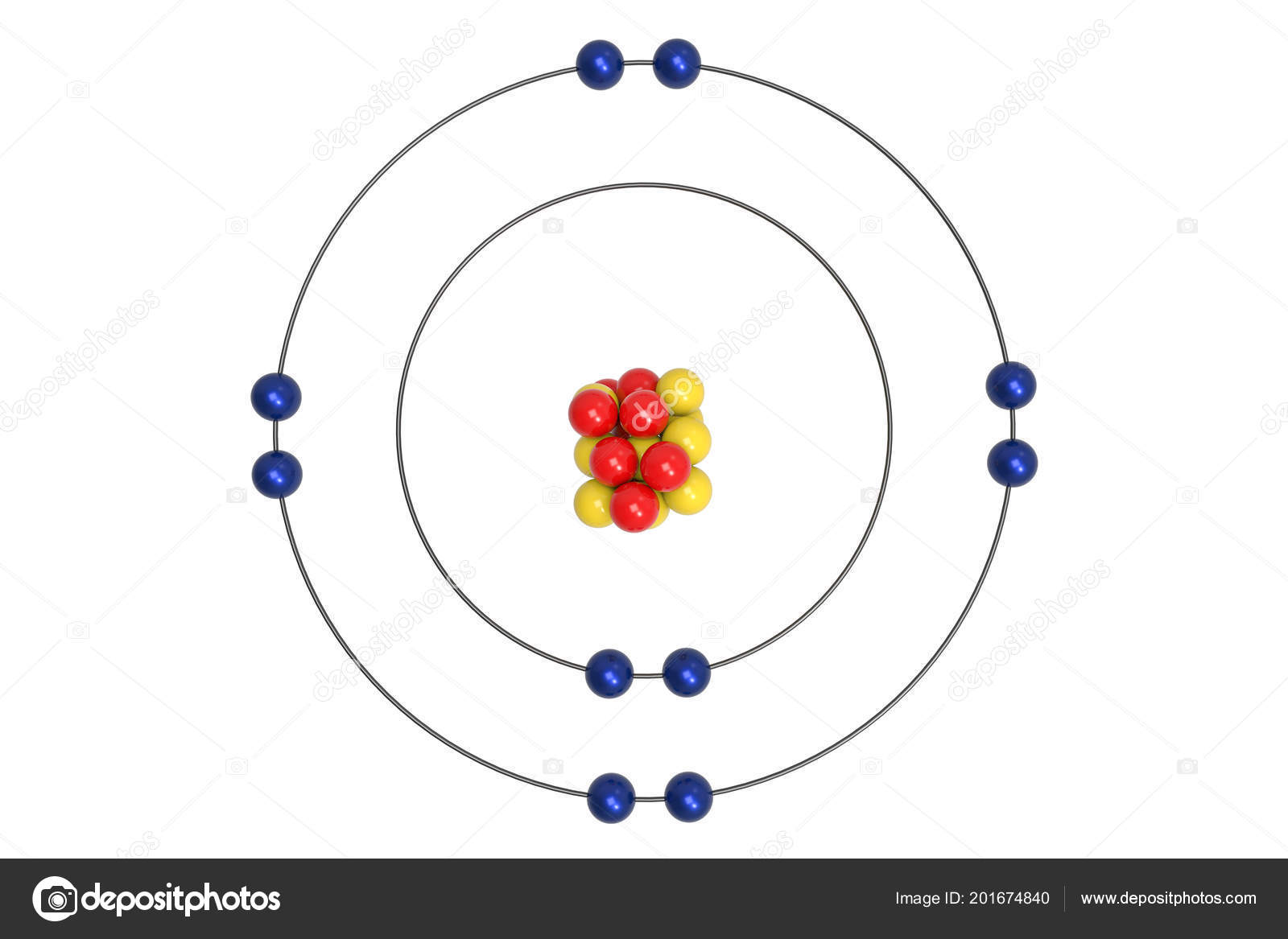
The Bohr Model of Neon (Ne) has a nucleus that contains 10 neutrons and 10 protons. This nucleus is surrounded by two-electron shells named K-shell and L-shell. The outermost shell in the Bohr diagram of Neon contains 8 electrons that also called valence electrons. Page Contents show How to draw Bohr Model of Neon (Ne)?
Neon Bohr Diagram
November 1, 2023 by Deep The information on this page is fact-checked. Neon Bohr model The Bohr model of neon contains a nucleus having 10 protons and 10 neutrons in the center, and around this nucleus, there are two electron shells containing 10 electrons. Atomic Structure (Bohr Model) for the Neon (Ne) Atom Watch on Contents Steps
Lewis Dot Diagram For Neon
Bohr diagrams show electrons orbiting the nucleus of an atom somewhat like planets orbit around the sun. In the Bohr model, electrons are pictured as traveling in circles at different shells, depending on which element you have. Figure 2 2 contrast the Bohr diagrams for lithium, fluorine and aluminum atoms. The shell closest to the nucleus is.

The Rutherford atomic model of a neon atom, showing the atom as similar
An example of a Bohr-Rutherford Diagram for oxygen is shown in Figure #2: Remember: Protons (p+) and neutrons (n0) are shown in the nucleus (centre) Electrons (red dots) are shown to orbit (move around) the nucleus in energy shells. Valence electrons are those that are in the outermost energy shell. Oxygen has 6 valence electron
modelo bohr del átomo de neón. vector de stock (libre de regalías
The Rutherford-Bohr atomic model is a representation of the atom as a very small. Neon Atomic # 10 Atomic mass # 20 10 electrons distributed like this: - 2 on the first level - 8 on the second level RUTHERFORD- BOHR DIAGRAM . VALENCE ELECTRONS

Neon Bohr model Science ShowMe
In atomic physics, the Bohr model or Rutherford-Bohr model of the atom, presented by Niels Bohr and Ernest Rutherford in 1913, consists of a small, dense nucleus surrounded by orbiting electrons.
Neon Atom Bohr Model Proton Neutron Electron Illustration Stock Photo
A detailed look at the Bohr -Rutherford Diagram for Phosphorus is provided below. The box on the left provides the Chemical Symbol (P), the Atomic. Neon Chlorine 40 Ca 20 11 B 5 20 Ne 10 35 Cl 17. Title: Unit 2 Lesson 5 Bohr-Rutherford Diagrams 2.PDF Author: Rm-227 Created Date:

neon atomic structure neon atom diagram QFB66
Bohr Model of Atoms. Following the work of Ernest Rutherford and his colleagues in the early twentieth century, the picture of atoms consisting of tiny dense nuclei surrounded by lighter and even tinier electrons continually moving about the nucleus was well established. This picture was called the planetary model, since it pictured the atom as a miniature "solar system" with the electrons.

Atomic Structure (Bohr Model) for the Neon (Ne) Atom YouTube
The Bohr Model is a modification of an earlier atomic model, the Rutherford Model. The Bohr Model has an atom with a positively-charged nucleus surrounded by negatively-charged electrons that have circular, planetary-like orbits. Today, we know that the Bohr Model has some inaccuracies, but it's still used because of its simple approach to.

Bohr Model Neon
Bohr model: energy levels. What is the ( i ) kinetic energy of the electron, and the ( ii ) potential energy of the atom in the state, n = 2 ? Stuck? Use a hint. Learn for free about math, art, computer programming, economics, physics, chemistry, biology, medicine, finance, history, and more. Khan Academy is a nonprofit with the mission of.